AI-Enhanced Simulations Provide New Insights into Proton Transport in Water
Using advanced molecular simulations powered by artificial intelligence, an international research team from École Normale Supérieure PSL, Sorbonne Université, CNRS, and the University of Kansas has uncovered critical aspects about how protons move through water, providing answers to longstanding questions in the field. These results were published on August 20, 2024 in the journal Nature Chemistry.
The movement of protons—positively charged hydrogen atoms—governs the conductivity of water and is vital in both biological processes, such as energy production in cells, and in artificial applications like fuel cells. For decades, scientists have known that protons move unusually quickly by transferring between water molecules, but recent nonlinear ultrafast laser experiments contradicted the established understanding based on traditional models. This left crucial questions about proton structure and diffusion unresolved.
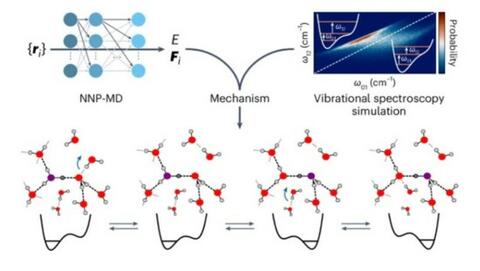
Researchers at ENS and KU tackled this challenge using cutting-edge, machine-learning-based molecular simulations. Their findings reveal that the mechanism of proton transport is more intricate than previously thought. While the excess proton is typically localized on a water molecule in the form of a hydronium ion (H₃O⁺), it is surrounded by distinct stable arrangements of the water’s hydrogen-bond network.
Given the timing of the recent Olympic Games, a sports analogy feels apt: proton diffusion can be compared to a basketball game. Just as the ball can either be possessed by one player or passed between players, the proton moves by two modes, either by regular ion diffusion or by the Grotthuss hopping between water molecules. However, the research team discovered that successful proton hopping requires specific conditions, much like a player needing a clear pass free from defenders. The “defenders” in this analogy represent hydrogen bonds that must break to allow a proton to transfer.
Moreover, the study emphasizes that after a proton is passed, the reformation of a hydrogen bond on the donor molecule is essential, akin to players repeatedly passing the ball until a decisive move is made. This sequential breakage and reformation of bonds create a stepwise diffusion process, in which the proton either stays on a single molecule or rapidly transfers between two.
This new understanding not only clarifies why proton diffusion rates behave unexpectedly at interfaces and within solids but also suggests innovative ways to control proton transport in various applications.
Reference: A. Gomez, W.H. Thompson, D. Laage “Neural-network-based molecular dynamics simulations reveal that proton transport in water is doubly gated by sequential hydrogen-bond exchange” Nature Chemistry
